In the realm of modern medicine, antibiotics have proven to be one of the most significant advancements, dramatically lowering the incidence and mortality rates associated with microbial infections. Their ability to alter the clinical outcomes of bacterial infections has extended the life expectancy of countless patients. Antibiotics are critical in complex medical procedures, including surgeries, implant placements, transplants, and chemotherapy. However, the emergence of antibiotic-resistant pathogens has been a growing concern, diminishing the efficacy of these drugs over time. Instances of antibiotic resistance have been documented across all categories of antibiotics as microbial mutations occur. The selection pressure exerted by antimicrobial drugs has contributed to the rise of resistant strains, posing a significant challenge to global health.
To combat the pressing issue of antimicrobial resistance, it is essential to implement effective infection control policies that curtail the spread of resistant pathogens, alongside reducing the utilization of antibiotics. Furthermore, there is a pressing need for alternative treatment methods. Hyperbaric Oxygen Therapy (HBOT) has emerged as a promising modality in this context, involving the inhalation of 100% oxygen at specific pressure levels for a period of time. Positioned as either a primary or complementary treatment for infections, HBOT may offer new hope in treating acute infections caused by antibiotic-resistant pathogens.
This therapy is increasingly applied as a primary or alternative treatment for various conditions, including inflammation, carbon monoxide poisoning, chronic wounds, ischemic diseases, and infections. The clinical applications of HBOT in infection treatment are profound, providing invaluable advantages to patients.

Clinical Applications of Hyperbaric Oxygen Therapy in Infection
Current evidence robustly supports the application of HBOT, both as a standalone and adjunctive treatment, presenting significant benefits to infected patients. During HBOT, arterial blood oxygen pressure can rise to 2000 mmHg, and the resultant high oxygen-tissue pressure gradient can elevate tissue oxygen levels to 500 mmHg. Such effects are particularly valuable in promoting the healing of inflammatory responses and microcirculatory disruptions observed in ischemic environments, as well as in managing compartment syndrome.
HBOT can also impact conditions reliant on the immune system. Research indicates that HBOT can suppress autoimmune syndromes and antigen-induced immune responses, helping to maintain graft tolerance by reducing the circulation of lymphocytes and leukocytes while modulating immune responses. Additionally, HBOT supports healing in chronic skin lesions by stimulating angiogenesis, a critical process for improved recovery. This therapy also encourages the formation of collagen matrix, an essential phase in wound healing.
Special attention must be given to certain infections, particularly deep and difficult-to-treat infections such as necrotizing fasciitis, osteomyelitis, chronic soft tissue infections, and infectious endocarditis. One of the most common clinical applications of HBOT is for skin-soft tissue infections and osteomyelitis associated with low oxygen levels that are often caused by anaerobic or resistant bacteria.
1. Diabetic Foot Infections
Diabetic foot ulcers are a prevalent complication among diabetic patients, affecting up to 25% of this population. Infections frequently arise in these ulcers (accounting for 40%-80% of cases) and lead to increased morbidity and mortality. Diabetic foot infections (DFIs) usually consist of polymicrobial infections with a variety of anaerobic bacterial pathogens identified. Various factors, including fibroblast function defects, collagen formation issues, cellular immune mechanisms, and phagocyte function, can hinder wound healing in diabetic patients. Several studies have identified impaired skin oxygenation as a strong risk factor for amputations related to DFIs.
As one of the current options for DFI treatment, HBOT has been reported to significantly enhance healing rates for diabetic foot ulcers, subsequently reducing the need for amputations and complicated surgical interventions. It not only minimizes the necessity for resource-intensive procedures, such as flap surgeries and skin grafting, but also presents lower costs and minimal side effects compared to surgical options. A study by Chen et al. demonstrated that more than 10 sessions of HBOT led to a 78.3% improvement in wound healing rates in diabetic patients.
2. Necrotizing Soft Tissue Infections
Necrotizing soft tissue infections (NSTIs) are often polymicrobial, typically arising from a combination of aerobic and anaerobic bacterial pathogens and are often associated with gas production. While NSTIs are relatively rare, they present a high mortality rate due to their rapid progression. Timely and appropriate diagnosis and treatment are key to achieving favorable outcomes, and HBOT has been recommended as an adjunctive method for managing NSTIs. Although there remains contention surrounding HBOT's use in NSTIs due to the lack of prospective controlled studies, evidence suggests that it may be correlated with improved survival rates and organ preservation in NSTI patients. A retrospective study indicated a significant reduction in mortality rates among NSTI patients receiving HBOT.
1.3 Surgical Site Infections
SSIs can be classified based on the anatomical site of the infection and can arise from various pathogens, including both aerobic and anaerobic bacteria. Despite advancements in infection control measures, such as sterilization techniques, the use of prophylactic antibiotics, and enhancements in surgical practices, SSIs remain a persistent complication.
One significant review has investigated the efficacy of HBOT in preventing deep SSIs in neuromuscular scoliosis surgery. Preoperative HBOT may significantly reduce the incidence of SSIs and facilitate wound healing. This non-invasive therapy creates an environment where oxygen levels in the wound tissues are elevated, which has been associated with the oxidative killing action against pathogens. Additionally, it addresses the lowered blood and oxygen levels that contribute to the development of SSIs. Beyond other infection control strategies, HBOT has been recommended particularly for clean-contaminated surgeries such as colorectal procedures.
1.4 Burns
Burns are injuries caused by extreme heat, electric current, chemicals, or radiation and can pose high morbidity and mortality rates. HBOT is beneficial in treating burns by increasing the oxygen levels in damaged tissues. While animal and clinical studies present mixed results regarding the effectiveness of HBOT in burn treatment, a study involving 125 burn patients indicated that HBOT showed no significant impact on mortality rates or the number of surgeries performed but did reduce average healing time (19.7 days compared to 43.8 days). Integrating HBOT with comprehensive burn management could effectively control sepsis in burn patients, leading to shorter healing times and reduced fluid requirements. However, further extensive prospective research is required to confirm the role of HBOT in the management of extensive burns.
1.5 Osteomyelitis
Osteomyelitis is an infection of the bone or bone marrow often caused by bacterial pathogens. Treating osteomyelitis can be challenging due to the relatively poor blood supply to bones and the limited penetration of antibiotics into the marrow. Chronic osteomyelitis is characterized by persistent pathogens, mild inflammation, and necrotic bone tissue formation. Refractory osteomyelitis refers to chronic bone infections that continue or recur despite appropriate treatment.
HBOT has been shown to significantly improve oxygen levels in the infected bone tissues. Numerous case series and cohort studies indicate that HBOT enhances clinical outcomes for osteomyelitis patients. It appears to work through various mechanisms, including boosting metabolic activity, suppressing bacterial pathogens, enhancing antibiotic effects, minimizing inflammation, and promoting healing processes. Post-HBOT, 60% to 85% of patients with chronic, refractory osteomyelitis show signs of infection suppression.
1.6 Fungal Infections
Globally, over three million individuals suffer from chronic or invasive fungal infections, leading to over 600,000 deaths annually. Treatment outcomes for fungal infections are often compromised due to factors like altered immune status, underlying diseases, and pathogen virulence characteristics. HBOT is becoming an attractive therapeutic option in severe fungal infections due to its safety and non-invasive nature. Studies indicate that HBOT could be effective against fungal pathogens such as Aspergillus and Mycobacterium tuberculosis.
HBOT promotes antifungal effects by inhibiting the biofilm formation of Aspergillus, with increased efficiency noted in strains lacking superoxide dismutase (SOD) genes. The hypoxic conditions during fungal infections pose challenges to antifungal drug delivery, making the increased oxygen levels from HBOT a potentially beneficial intervention, although further research is warranted.
The Antimicrobial Properties of HBOT
The hyperoxic environment created by HBOT initiates physiological and biochemical changes that stimulate antibacterial properties, making it an effective adjunct therapy for infection. HBOT demonstrates remarkable effects against aerobic bacteria and predominantly anaerobic bacteria through mechanisms such as direct bactericidal activity, enhancement of immune responses, and synergistic effects with specific antimicrobial agents.
2.1 Direct Antibacterial Effects of HBOT
The direct antibacterial effect of HBOT is largely attributed to the generation of reactive oxygen species (ROS), which include superoxide anions, hydrogen peroxide, hydroxyl radicals, and hydroxyl ions—all of which arise during cellular metabolism.
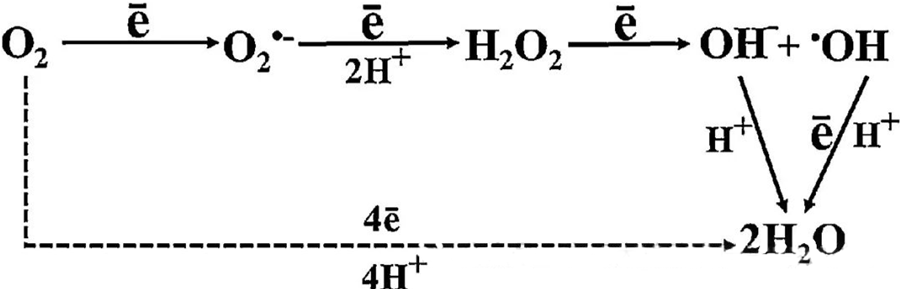
The interaction between O₂ and cellular components is essential in understanding how ROS forms within cells. Under certain conditions referred to as oxidative stress, the balance between ROS formation and its degradation is disrupted, leading to elevated levels of ROS in cells. The production of superoxide (O₂⁻) is catalyzed by superoxide dismutase, which subsequently converts O₂⁻ into hydrogen peroxide (H₂O₂). This conversion is further amplified by the Fenton reaction, which oxidizes Fe²⁺ to generate hydroxyl radicals (·OH) and Fe³⁺, thus initiating a detrimental redox sequence of ROS formation and cellular damage.
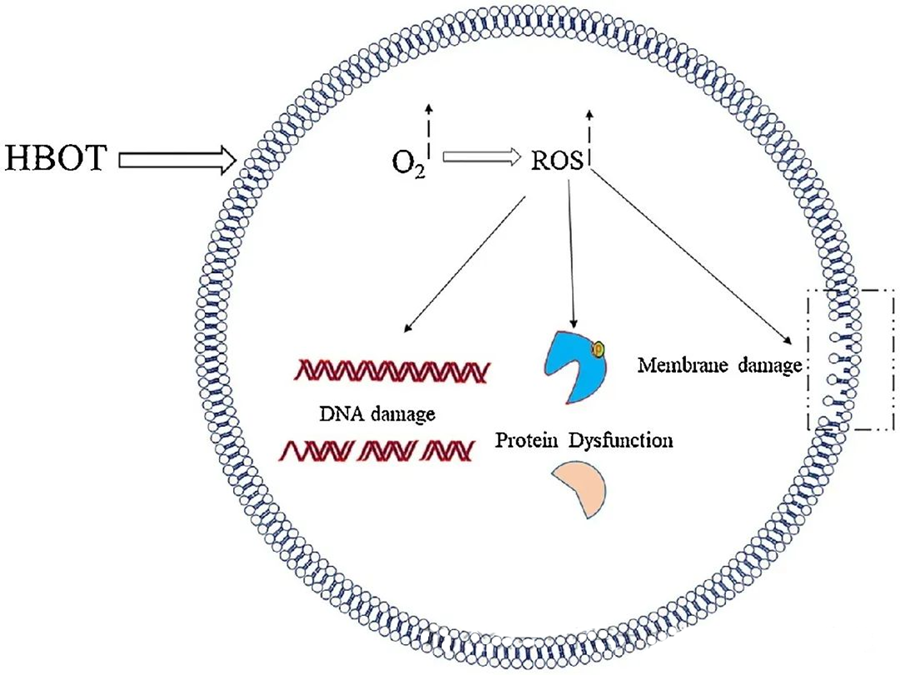
The toxic effects of ROS target critical cellular components such as DNA, RNA, proteins, and lipids. Notably, DNA is a primary target of H₂O₂-mediated cytotoxicity, as it disrupts deoxyribose structures and damages base compositions. The physical damage induced by ROS extends to the helix structure of DNA, potentially resulting from lipid peroxidation triggered by ROS. This underscores the adverse consequences of elevated ROS levels within biological systems.
Antimicrobial Action of ROS
ROS play a vital role in inhibiting microbial growth, as demonstrated through HBOT-induced ROS generation. The toxic effects of ROS directly target cellular constituents like DNA, proteins, and lipids. High concentrations of active oxygen species can directly damage lipids, leading to lipid peroxidation. This process compromises the integrity of cell membranes and, consequently, the functionality of membrane-associated receptors and proteins.
Furthermore, proteins, which are also significant molecular targets of ROS, undergo specific oxidative modifications at various amino acid residues such as cysteine, methionine, tyrosine, phenylalanine, and tryptophan. For instance, HBOT has been shown to induce oxidative changes in several proteins in E. coli, including elongation factor G and DnaK, thereby affecting their cellular functions.
Enhancing Immunity Through HBOT
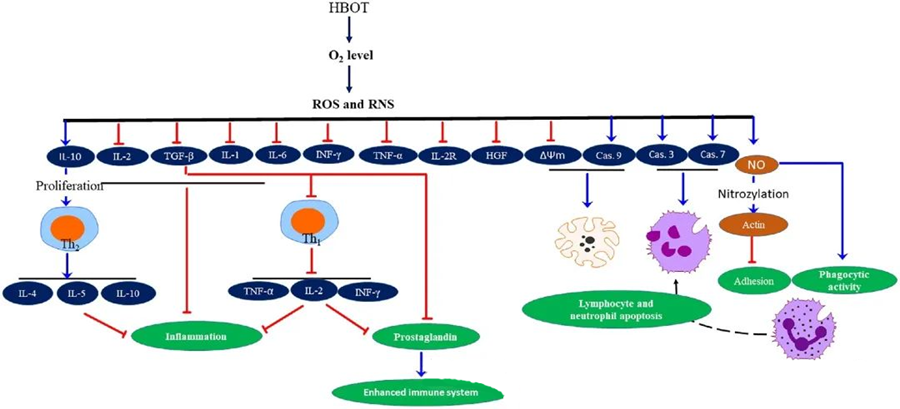
The anti-inflammatory properties of HBOT have been documented, proving crucial for alleviating tissue damage and suppressing infection progression. HBOT significantly impacts the expression of cytokines and other inflammatory regulators, influencing the immune response. Various experimental systems observed differential changes in gene expression and protein generation post-HBOT, which either upregulate or downregulate growth factors and cytokines.
During the HBOT process, increased O₂ levels trigger a range of cellular responses, such as suppressing the release of pro-inflammatory mediators and promoting lymphocyte and neutrophil apoptosis. Collectively, these actions enhance the immune system's antimicrobial mechanisms, thereby facilitating the healing of infections.
Furthermore, studies suggest that increased O₂ levels during HBOT can reduce the expression of pro-inflammatory cytokines, including interferon-gamma (IFN-γ), interleukin-1 (IL-1), and interleukin-6 (IL-6). These changes also include downregulating the ratio of CD4:CD8 T cells and modulating other soluble receptors, ultimately raising interleukin-10 (IL-10) levels, which is crucial for counteracting inflammation and fostering healing.
The antimicrobial activities of HBOT are intertwined with complex biological mechanisms. Both superoxide and elevated pressure have been reported to inconsistently promote HBOT-induced antibacterial activity and neutrophil apoptosis. Following HBOT, a marked elevation in oxygen levels enhances the bactericidal capabilities of neutrophils, an essential component of the immune response. Furthermore, HBOT suppresses neutrophil adhesion, which is mediated by the interaction of β-integrins on neutrophils with intercellular adhesion molecules (ICAM) on endothelial cells. HBOT inhibits the activity of neutrophil β-2 integrin (Mac-1, CD11b/CD18) through a nitric oxide (NO)-mediated process, contributing to the migration of neutrophils to the site of infection.
The precise rearrangement of the cytoskeleton is necessary for neutrophils to effectively phagocytize pathogens. S-nitrosylation of actin has been shown to stimulate actin polymerization, potentially facilitating the phagocytic activity of neutrophils after HBOT pre-treatment. Moreover, HBOT promotes apoptosis in human T cell lines through mitochondrial pathways, with accelerated lymphocyte death post-HBOT being reported. Blocking caspase-9—without impacting caspase-8—has demonstrated the immunomodulatory effects of HBOT.
The Synergistic Effects of HBOT with Antimicrobial Agents
In clinical applications, HBOT is frequently used alongside antibiotics to combat infections effectively. The hyperoxic state achieved during HBOT can influence the efficacy of certain antibiotic agents. Research suggests that specific bactericidal drugs, such as β-lactams, fluoroquinolones, and aminoglycosides, not only act through inherent mechanisms but also rely partially on the aerobic metabolism of bacteria. Therefore, the presence of oxygen and the metabolic characteristics of pathogens are pivotal when evaluating the therapeutic effects of antibiotics.
Significant evidence has shown that low oxygen levels can increase the resistance of Pseudomonas aeruginosa to piperacillin/tazobactam and that a low oxygen environment also contributes to the increased resistance of Enterobacter cloacae to azithromycin. Conversely, certain hypoxic conditions may enhance bacterial sensitivity to tetracycline antibiotics. HBOT serves as a viable adjunctive therapeutic method by inducing aerobic metabolism and reoxygenating hypoxic infected tissues, subsequently increasing the sensitivity of pathogens to antibiotics.
In preclinical studies, the combination of HBOT—administered twice daily for 8 hours at 280 kPa—alongside tobramycin (20 mg/kg/day) significantly reduced bacterial loads in Staphylococcus aureus infectious endocarditis. This demonstrates the potential of HBOT as an auxiliary treatment. Further investigations have revealed that under 37°C and 3 ATA pressure for 5 hours, HBOT notably enhanced the effects of imipenem against macrophage-infected Pseudomonas aeruginosa. Additionally, the combined modality of HBOT with cephazolin was found to be more effective in treating Staphylococcus aureus osteomyelitis in animal models compared to cephazolin alone.
HBOT also significantly increases the bactericidal action of ciprofloxacin against Pseudomonas aeruginosa biofilms, particularly following 90 minutes of exposure. This enhancement is attributed to the formation of endogenous reactive oxygen species (ROS) and displays heightened sensitivity in peroxidase-defective mutants.
In models of pleuritis caused by methicillin-resistant Staphylococcus aureus (MRSA), the collaborative effect of vancomycin, teicoplanin, and linezolid with HBOT showed significantly increased efficacy against MRSA. Metronidazole, an antibiotic extensively utilized in treating severe anaerobic and polymicrobial infections such as diabetic foot infections (DFIs) and surgical site infections (SSIs), has exhibited higher antimicrobial effectiveness under anaerobic conditions. Future studies are warranted to explore the synergistic antibacterial effects of HBOT combined with metronidazole in both in vivo and in vitro settings.
The Antimicrobial Efficacy of HBOT on Resistant Bacteria
With the evolution and spread of resistant strains, traditional antibiotics often lose their potency over time. Furthermore, HBOT may prove essential in treating and preventing infections caused by multidrug-resistant pathogens, serving as a critical strategy when antibiotic treatments fail. Numerous studies have reported the significant bactericidal effects of HBOT on clinically relevant resistant bacteria. For instance, a 90-minute HBOT session at 2 ATM substantially reduced the growth of MRSA. Additionally, in ratio models, HBOT has enhanced the antibacterial effects of various antibiotics against MRSA infections. Reports have confirmed that HBOT is effective in treating osteomyelitis caused by OXA-48-producing Klebsiella pneumoniae without necessitating any adjunctive antibiotics.
In summary, hyperbaric oxygen therapy represents a multifaceted approach to infection control, enhancing the immune response while also amplifying the efficacy of existing antimicrobial agents. With comprehensive research and development, it holds the potential to mitigate the effects of antibiotic resistance, offering hope in the ongoing battle against bacterial infections.
Post time: Feb-28-2025

